Wasser und Umwelt - Age dating of groundwater
Groundwater forms from precipitation and infiltration of surface water. Once underground, it gives us no insight about its underground flow paths. It can travel long distances or return immediately to the surface via wells and springs. Isotope ratios provide valuable information about the age and origin of the water.
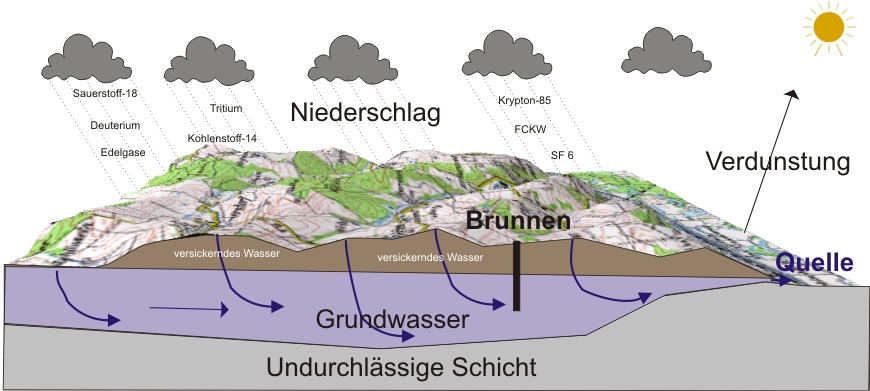
Oxigen-18 (18O) and Deuterium (2H) provide information about the origin and formation conditions of groundwater such as altitude or climate.
Tritium(3H) by itself does not provide clear and detailed information on groundwater age. A tritium concentration <0.1 TU means that the water must have been formed before 1950.
Krypton-85(85Kr) in conjunction with tritium provides information on the age of relatively young waters. By combining and interpreting these two isotopes, a mixture of old and young groundwater can easily be identified.
Helium-3(3He) can also be combined with tritium. A detection accuracy of a few years can be achieved when using the 3H - 3He method for determining the age of groundwater.
Kohlenstoff-14 (14C) is suitable for the determination of old groundwater due its half-life of 5730 years.
Krypton-81 is suitable to detect very old water in the range from 50,000 years ago until about 1 million years to date.
Sulfur hexafluoride (Sf6) provides an economical alternative for dating groundwater that is younger than 10 years.
